In thermodynamics thermal energy also called the internal energy is defined as the energy associated with microscopic forms of energy. The customary unit is called the Btu or British thermal unit.
Vocabulary Thermal Energy Heat Calorie Specific Heat Ppt Video Online Download
In the United States Btu a measure of heat energy is the most common unit for comparing energy sources or fuels.
. The work and heat are also forms of energy. -The unit often used in chemistry and physics to measure energy such as for work or electrical current is the _____-We can also use this unit as we did in this lab for measuring _____. Petajoule picojoule SI unit.
This lets you compare the amount of thermal energy in two different things. Notably heat is a form of energy and therefore the SI unit of heat is also joules J which are defined as the amount of energy needed to raise the temperature of a given mass by one degree. It is an extensive quantity it depends on the size of the system or on the amount of substance it contains.
To determine the thermal expansion coefficient two physical quantities displacement and temperature must be measured on a sample that is undergoing a thermal cycle. 11 International System of Units Simple English Wikipedia the free. Heat is the flow of thermal energy.
Three of the main techniques used for CTE measurement are dilatometry interferometry and thermomechanical analysis. The transfer of thermal energy by the movement of currents within a liquid or a gas. The French thermal unit or kilogram calorie is the quantity of heat or thermal energy required to raise the temperature of one kilogram of pure water one degree C.
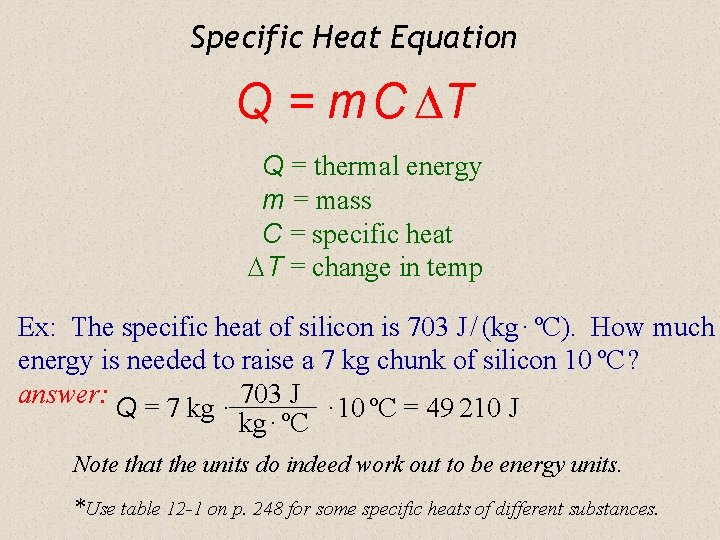
So the simplest way to measure thermal energy is to take a glass tube and fill it up with some kind of liquid. Therefore the relationship between commercial and SI unit of energy is. Where Q is the thermal energy transferred C th is the thermal mass of the body and ΔT is the change in temperature.
Based on what you have learned about the units for measuring thermal energy complete the following passage. This is a property that is quantified by the energy needed per of material to raise the temperature by one. The unit of heat in the imperial system - the BTU - is.
2 What unit do you use to measure thermal energy quizlet. The unit often used in chemistry and physics to measure energy such as for work or electrical current is the. 1 calorie 4184 joules J 1 thermie 1 million calories.
Hence the units of measurement of energy work and heat are same. It is the quantity of heat required to raise the temperature of one pound of liquid water by 1 degree Fahrenheit at the temperature that water has its greatest density approximately 39 degrees Fahrenheit. However the 12 ounce cup has twice as many molecules when compared with the 6.
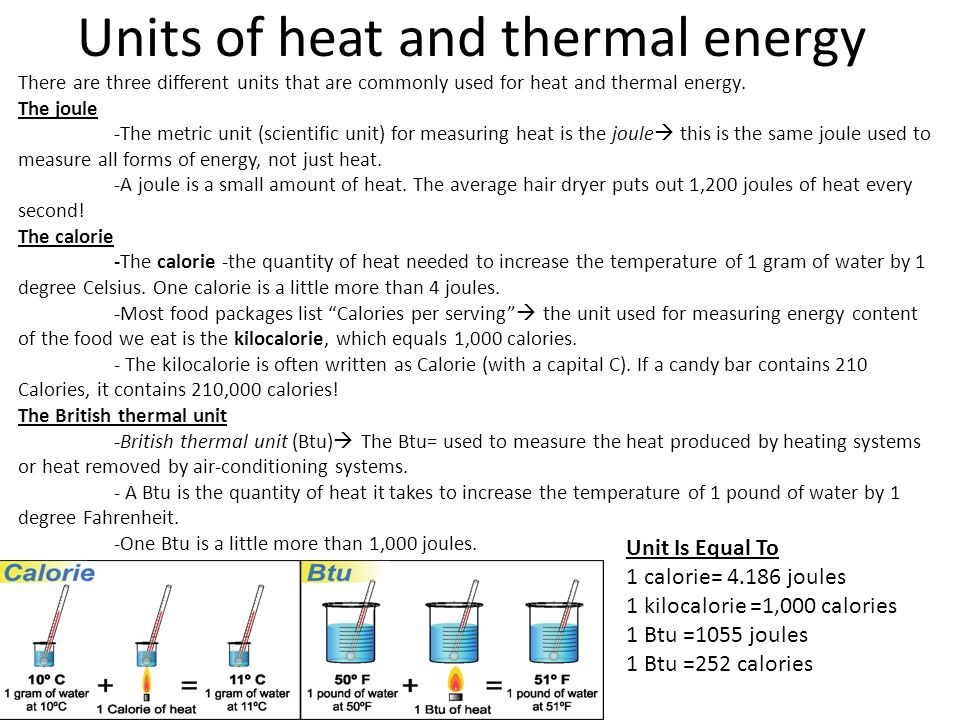
Units 1000 gram calories. The SI unit of thermal energy is the joule J. The most common units for heat are.
A British thermal unit Btu is a measure of the heat content of fuels or energy sources. A six ounce cup and a twelve ounce cup are both filled with 85 degree water. The joule is the standard metric unit for measuring any type of energy including heat.
One Btu was originally defined as the quantity of heat required to raise the temperature of 1 lb 0. Some popular units for comparing energy include British thermal units Btu barrels of oil equivalent metric tons of oil equivalent metric tons of coal equivalent and terajoules. The amount of heat required to raise the temperature of one pound of water through 1 o F 585 o F - 595 o F at sea level 30.
British Thermal Unit a unit measurement of heat or energy usually abbreviated as Btu or BTU. The unit of heat energy is Joule though the older unit of calorie is also used. 1 What Is The Unit Used To Measure Thermal Energy.
The SI unit of energy is Joule. A measure of the average kinetic energy of a substance. British Thermal Unit BTU Imperial unit of measurement for energy.
A total energy of molecular motion in a substance. 1 BTU 1055 joules J Calorie cal thermie th Former unit of measurement for energy. They do NOT have the same heat contentSince water in the two cups is at the same temperature the average kinetic energy of the molecules in the cups is the same.
One kilowatt-hour is defined as the amount of energy consumed by a device in one working hour at a constant rate of one kilowatt. 1 kWh 1kW x 1h 1000W x 1h 1000Js x 3600 s 36 x10 6 J. The unit for this measure is therefore.
BTU - British Thermal Unit. For example if 250 J of heat energy is added to a copper gear with a thermal mass of 3846 JC its temperature will rise by 650 C. The number of foot-pounds of mechanical energy equivalent to one British thermal unit is called the.
BTU Btu - British Thermal Unit - also known as a heat unit in United States. We can also use this unit as we did in this lab for measuring. Thermal energy refers to the energy contained within a system that is responsible for its temperature.
One British thermal unit Btu is approximately. 19 rows 1 barrel of oil equivalent corresponds to the energy produced when a barrel of oil is burned. There are other units of measure for energy that are used throughout the world including kilowatt-hours calories newton-meters therms.
The commercial unit of energy is 1 kWh. Optical imaging can also be used at extreme temperatures. Units for comparing energy.
We can also use this unit as we did in this lab for measuring. It is the energy contained within the system. Energy is defined as the ability to do work ie.
Learn vocabulary terms and more with flashcards games and other study tools. 3 How do you measure thermal. One kilogram calorie 3968 British thermal.
Based on what you have learned about the units for measuring thermal energy complete the following passage. The unit often used in chemistry and physics to measure energy such as for work or electrical current is the. Start studying Thermal Energy Unit.
The equation relating thermal energy to thermal mass is. As the tube heats up the liquid heats up and expands and rises in the tube. A whole branch of physics thermodynamics deals with how heat is transferred between different systems and how work is done in the process see the 1ˢᵗ law of thermodynamics.
As per the second law of thermodynamics whenever the work is done by absorbing the energy heat from the reservoir some heat is always rejected to the sink. 4 What unit is used to measure the thermal energy that flows from an object of a higher temperature to an object of a lower temperature. Usually 4184 joules of heat energy is necessary to increase the temperature of a unit weight say 1 g of water from 0 degrees to 1 degree Celsius.
This is a property that is quantified by the energy needed per. The metric unit is the joule.

What Is Thermal Energy Article Khan Academy

Investigation 9a Key Question How Are Temperature And Heat Related Temperature And Heat Ppt Download

Heat A Form Of Energy Thermal Energy 12

Lesson 1 Thermal Energy Temperature And Heat Ppt Video Online Download
0 Comments